Kent State to pause all distribution of the Johnson & Johnson vaccines
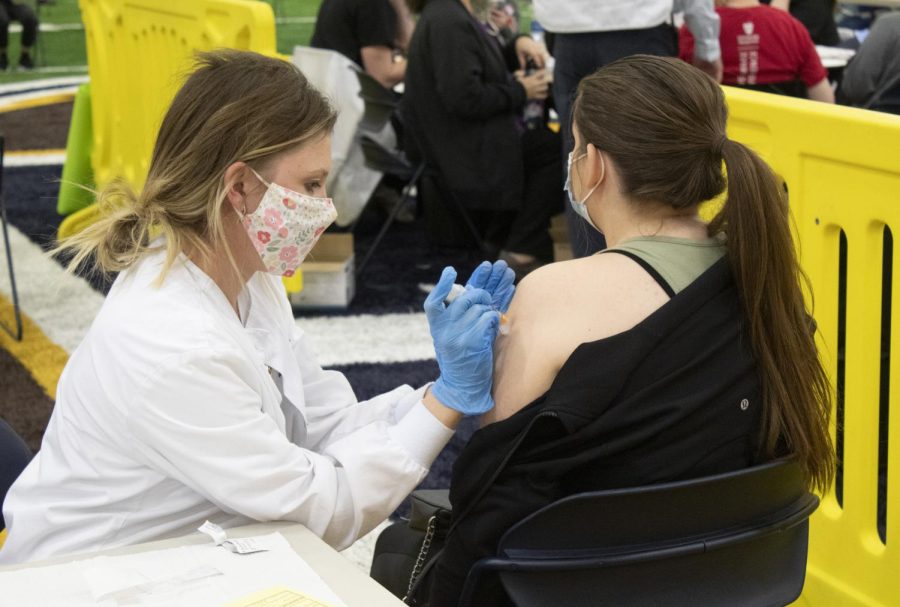
Senior nursing student Courtney Shutt administers the first dose of the Moderna vaccine to senior sport administration major Ginny Weavill on March 30, 2021. Following April 13’s recommendation by the Centers for Disease Control and Prevention (CDC) and the Food and Drug Administration (FDA) to pause the administration of the Johnson & Johnson single-dose COVID-19 vaccine, Kent State paused the distribution of this vaccine to students, which began April 8.
Kent State is postponing all distribution of the Johnson & Johnson vaccines due to recommendations by the CDC and FDA.
This decision follows the announcement from the CDC and FDA stating they are currently reviewing data from the Johnson & Johnson vaccine due to six reported cases of a severe blood clot happening post-vaccination and recommend pausing distribution.
Due to this recommendation, Kent State has postponed all clinics scheduled to distribute the Johnson & Johnson vaccine until further notice.
These six cases happened to women between the ages of 18 and 48, with symptoms occurring six to 13 days after their vaccination. While this is extremely rare, the CDC and FDA recommend those who develop severe headaches, abdominal pain, leg pain or shortness of breath within three weeks of their Johnson and Johnson vaccine should reach out to their doctors.
As the CDC and FDA work to review the data from the Johnson and Johnson vaccine, they “are recommending a pause in the use of this vaccine out of an abundance of caution,” stated the CDC and FDA’s announcement.
The Johnson & Johnson vaccine was being administered in an effort to vaccinate all college students before the summer break as provided by the state of Ohio.
“Gov. Mike DeWine also has asked all Ohio vaccine providers to halt the use of the Johnson & Johnson shot,” an email from Kent State stated.
Those who may have had an appointment scheduled to get the Johnson and Johnson vaccine are encouraged to schedule an appointment to receive another brand of vaccine, Moderna or Pfizer, at a different clinic, including clinics operated by the Portage County Combined General Health District on Tuesdays at the Kent State Field House.
Contact Sara Crawford at [email protected].